2026 Predictions: Future Regulatory Impacts on LTC Pharmacies
Regulation in long term care (LTC) pharmacy has always evolved alongside technology, but 2026 is poised to be a defining year. From AI compliance and data privacy to controlled substance monitoring and pharmacist oversight, new and proposed rules are expected to reshape how LTC pharmacies manage operations, documentation, and patient safety.
AI Compliance Takes Center Stage
The rapid adoption of artificial intelligence in healthcare is drawing increased attention from regulators. Several states, including Colorado, Utah, and California, have enacted or proposed AI-specific laws focused on transparency, accountability, and data privacy.
At the federal level, policymakers are debating how much authority states should have in enforcing AI-related regulations. Meanwhile, in August 2025, the U.S. Food and Drug Administration (FDA) issued updated guidance on “Predetermined Change Control Plans” (PCCPs) for machine learning-enabled medical devices, offering a framework for how AI systems can evolve safely while maintaining regulatory oversight.
Across all levels of regulation, three key themes are emerging: explainability, transparency, and governance.

Predicted Impacts for 2026
- AI Standards: AI-powered pharmacy systems will need to meet clinical-grade standards with documented accuracy testing, explainability, and audit trails to verify compliance.
- Transparency Requirements: Regulators will require that AI-driven outputs remain reviewable by licensed pharmacists, ensuring human oversight for all patient-impacting decisions.
- Data Governance: HIPAA-aligned frameworks will expand to include AI data lifecycle management, from model training to inference and data storage.
As with early privacy laws, AI governance is likely to evolve unevenly across states until a federal standard emerges. LTC pharmacies will face increasing complexity and must stay informed about both state and national AI healthcare regulations.
Stengthened Data Privacy and Security Regulations
With increasing reliance on cloud-based pharmacy management systems, data privacy and cybersecurity remain top regulatory priorities in 2026.
Expected Developments
- Tighter HIPAA Enforcement:
Expect closer scrutiny on EHR integrations, e-prescribing systems, and data-sharing practices. - Interoperability Mandates:
Federal efforts to improve healthcare coordination will push for secure, seamless data exchange between pharmacies and providers. - Real-Time Compliance Audits:
Pharmacies may soon need to provide near-live access logs for all clinical and dispensing transactions.
FrameworkInsight supports these goals by offering centralized, auditable data access and AI-powered alerts that detect anomalies or potential security risks in real time.
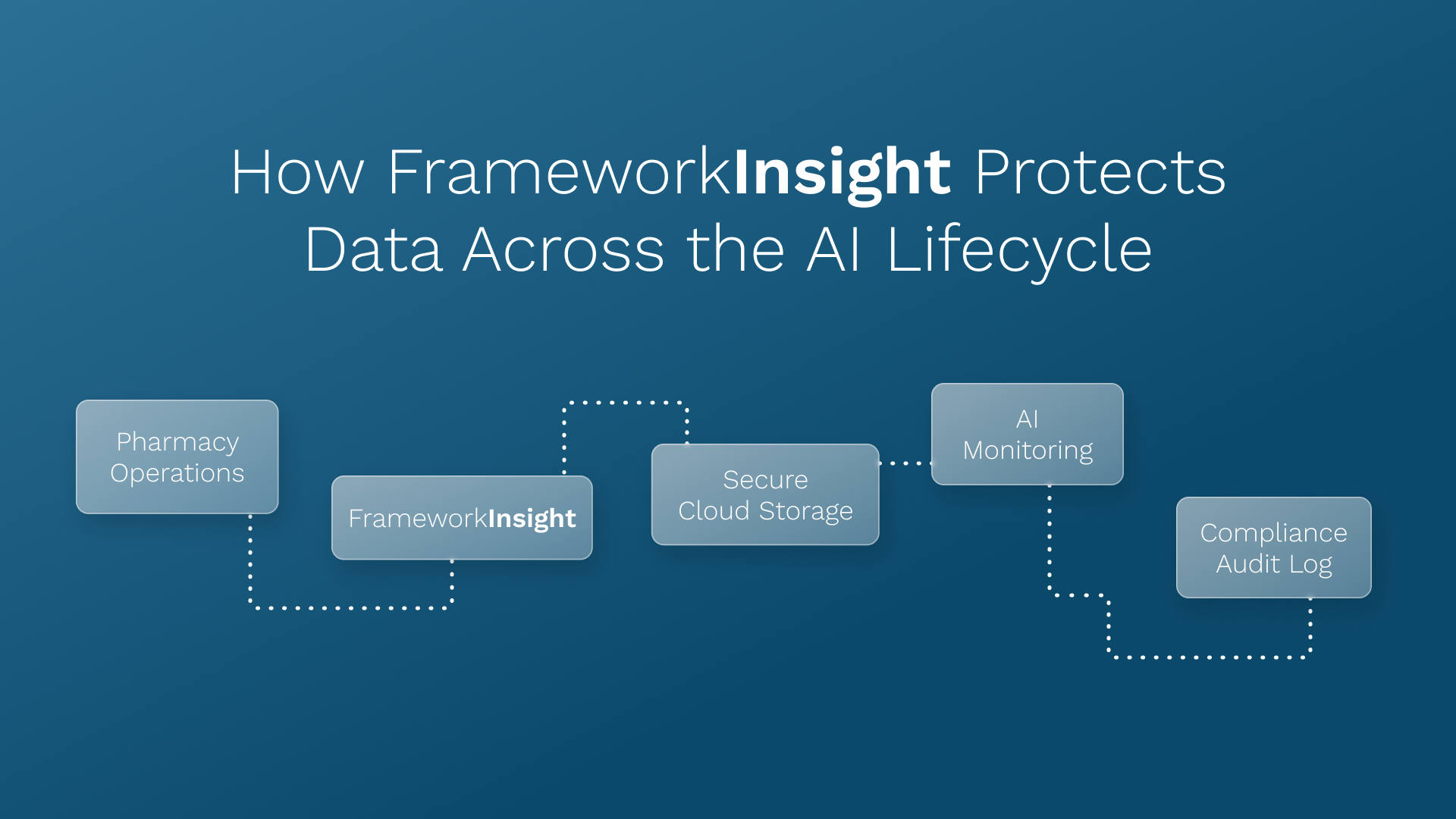
Medication Management and Clinical Oversight Reform
As patient safety continues to be a priority, regulators will expand requirements around medication tracking, reconciliation, and controlled substance management.
Likely 2026 Changes
- Electronic prescribing of controlled substances (EPCS) will become mandatory for most, with LTC pharmacy inclusion by January 1, 2028
- Enhanced medication reconciliation reporting will require pharmacies to document adherence and error-reduction strategies in real time.
- AI-enabled quality monitoring may become a compliance tool, using predictive analytics to flag anomalies before they escalate into violations.
FrameworkInsight’s KPI dashboards already align with this direction, allowing pharmacies to track fill rates, error trends, and turnaround times in compliance-ready formats.
Pharmacist Oversight and Certification Expansion
AI is transforming the daily work of pharmacists, but regulators will ensure that human oversight remains mandatory.
Expected Updates
- Mandatory Review Protocols:
All AI-generated orders and recommendations must be verified by a licensed pharmacist. - Certification Standards:
New training or certification may be required for pharmacists managing AI-enabled systems. - Ethical AI Oversight:
Regulations will reinforce pharmacist accountability for all final clinical decisions.
FrameworkLTC’s AI solutions are already built on a human-in-the-loop model, where pharmacists maintain control and transparency over every AI-supported action.
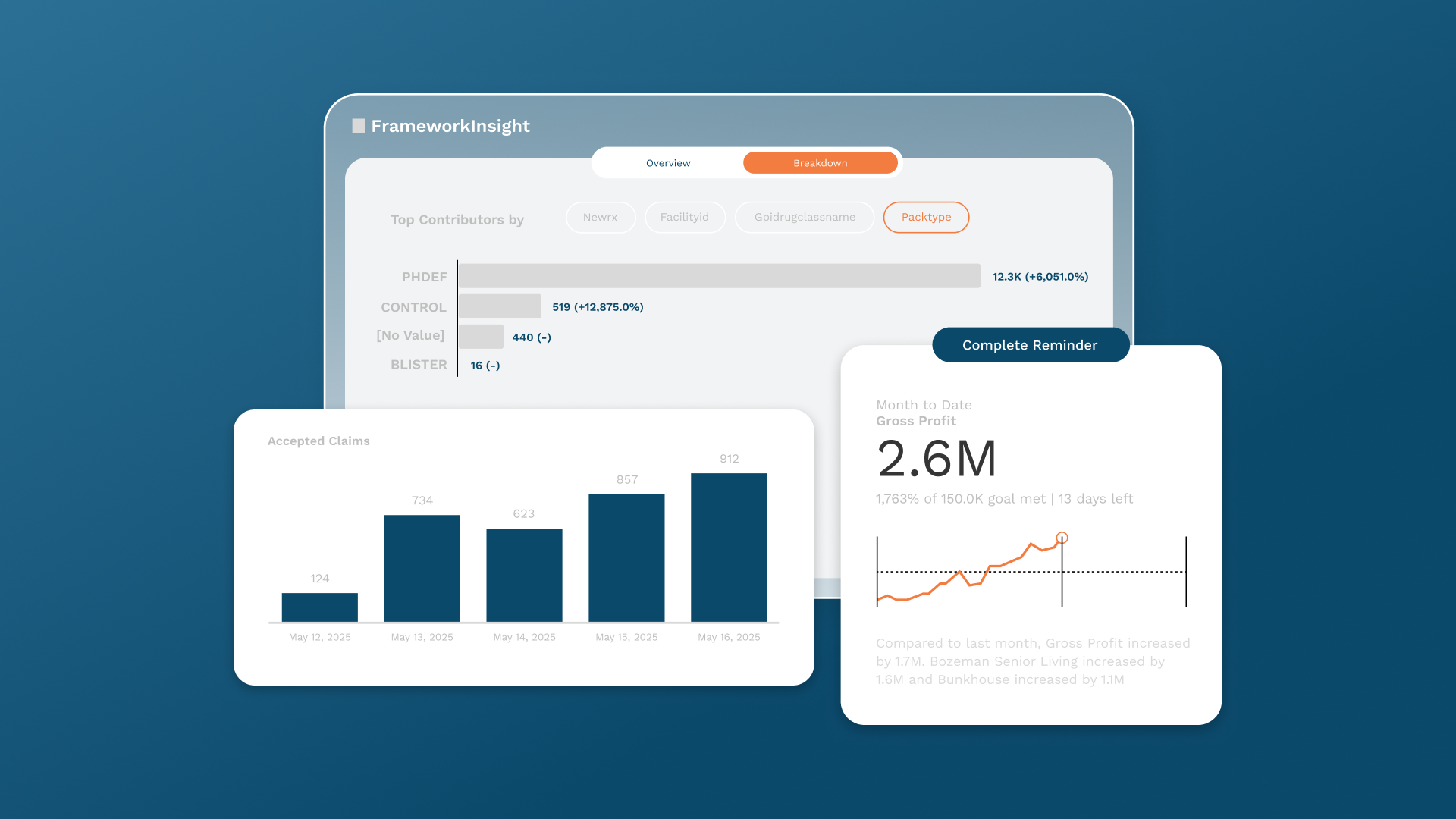
The 2026 Compliance Road Ahead
The pace of healthcare regulation is accelerating, and the LTC pharmacies that thrive will be those equipped to adapt in real time. Staying ahead requires maintaining clear visibility into operations and compliance metrics, leveraging AI-driven dashboards that automatically track and document key performance indicators, and partnering with technology providers who deeply understand the evolving regulatory landscape of long-term care pharmacy.
FrameworkLTC continues to lead this charge, investing in solutions that make compliance an integrated, automated part of everyday operations rather than an afterthought. As 2026 approaches, FrameworkLTC remains the partner that helps pharmacies prepare, not react.
.png?width=352&name=Your%20paragraph%20text%20(Presentation).png)

